Metabolism
Metabolism is the body’s process of breaking down almost any materials that enter it. The body metabolizes food, transforming it into useful energy and nutrients. The body metabolizes harmful foreign compounds from the outside environment, breaking them down into less harmful particles called metabolites that can then exit the body. The body also metabolizes drugs, and most drugs are metabolized into less active or inactive metabolites.
Although metabolism happens throughout the body, most of metabolism occurs in the liver. When a drug reaches the liver, it comes into contact with liver enzymes. These enzymes help facilitate specific chemical reactions. In general, most drugs are lipophilic, or fat-loving. These chemical reactions change the drug from being more lipophilic, into being more hydrophilic (water-loving). As a drug is transformed into its more hydrophilic metabolites, it becomes easier for the drug to exit the body via
excretion.
1 Cytochromes P450 (CYP) are an important family of metabolizing enzymes, that are located in the liver.
2, 3 Some other significant sites where metabolism occurs are the gastrointestinal (GI) tract, the kidneys, and the lungs.
4
Prodrugs
However, the metabolism process can also turn an inactive drug into an active metabolite. These types of drugs are called prodrugs. For prodrugs, the metabolites are actually the active therapeutic compounds. An example of this is cyclophosphamide, an inert compound which the body metabolizes into a powerful anti-cancer drug.
1 This phenomenon is the reason why some researchers prefer to explain metabolism as ‘biotransformation’ and not as a process of ‘breaking down’
Metabolic Drug-Drug interaction and Food-Drug interaction
It’s very common for patients to be treated by more than one drug at a time. However, it’s important to be mindful of drug-drug interactions, and even food-drug interactions. When a drug is in the presence of another drug, or a specific food, the concentration of these drugs can be altered. There are various types of drug-drug interactions, including interactions on the level of drug
absorption (e.g. one drug increases the ability of the other drug to be absorbed), metabolism, and there are even effect-interactions that can take place on multiple levels. In this section, we only focus on drug-drug interaction on a metabolic level.
Drug-drug and food-drug interactions can be caused by
enzyme inhibition (decreasing the speed of metabolism) and by enzyme induction (increasing the speed of metabolism). For example, a patient may be taking drug A and drug B. In the case of enzyme inhibition, if drug A and drug B are both competing for a site on the enzyme, only one will eventually bind to the enzyme – let’s say drug A. The enzyme will then predominantly metabolize drug A before B gets a chance to be metabolized. In this example, even if the patient only took one pill of drug B, the patient could experience the effects of two pills of drug B due to the slowed down metabolic interaction with drug A. See Figure 1 for this concept.
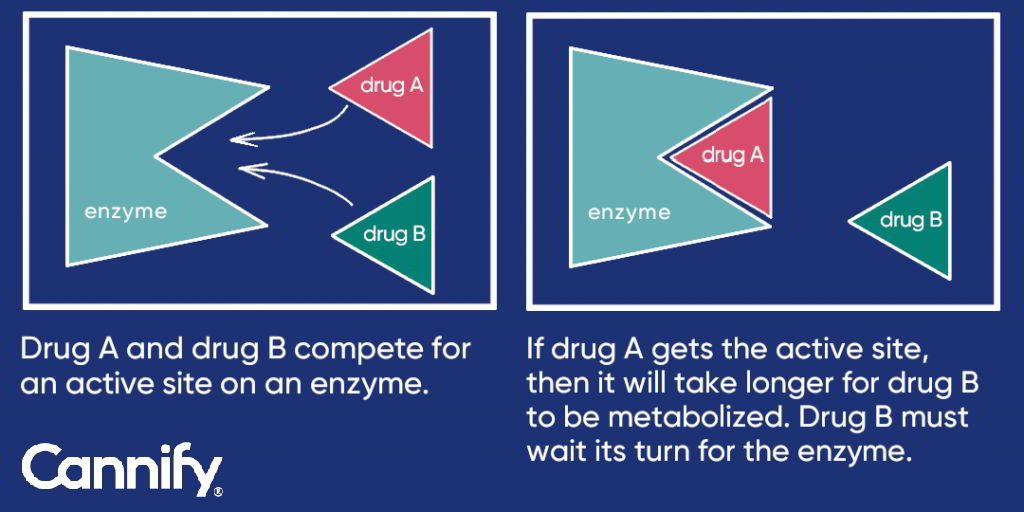 Figure 1: Enzyme inhibition – Drug A and drug B competition illustration.
Figure 1: Enzyme inhibition – Drug A and drug B competition illustration.
In the case of
enzyme induction, both drug C and drug D would be able to bind to the enzyme. However, drug C would act as an enzyme inducer, and make the enzyme metabolize drug D faster than usual. In this example, even if the patient only took one pill of drug D, the patient could experience the effects of only half a pill of drug D due to the accelerated metabolic interaction with drug C.
Drug metabolic interactions can cause several different effects in a patient. You might also have heard about interactions between
THC and
CBD (e.g. memory-impairing effects of THC are mitigated by CBD, when both are taken together
5), or about ‘the entourage effect’. These effects are not primarily caused by metabolic interaction, and are discussed in different sections on this education page.
First-pass effect
The first-pass effect is a phenomenon that occurs when a drug is orally administered. During this phenomenon, the drug will travel through the GI tract, and then to the liver where it is metabolized. After exiting the liver, the drug will finally enter the central bloodstream, which will distribute the metabolites and original drug (parent compound) throughout the body. Because of this process, the concentration of the drug and its metabolites in the bloodstream is different, compared to other administration methods. For example, taking a drug via IV (intravenous) injection immediately delivers the drugs into the body’s central bloodstream. With a drug that has been inhaled, it can immediately enter the bloodstream through the lungs. Therefore, the same dose of a drug taken through different administration methods will produce different effects.
In humans, delta9-tetrahydrocannabinol (THC) is mainly metabolized (see Figure 2) via the cytochrome P450 (CYP) enzyme system, which contains various enzymes. The chemical processes that happen during the breakdown of THC are called hydroxylation {call-out, text:Introducing a hydroxyl (OH) group into the compound which converts lipophilic compounds into water-soluble products that are more readily removed by the kidneys or liver and excreted from the body.} and oxidation {call-out, text:Interaction between oxygen molecules and other substances.} and are induced by enzymes that include CYP2C9 and CYP3A, to a lesser extent CYP2C19, and others.
2, 3
THC follows a certain path as it is metabolized by enzymes. Initially, THC is converted into 11-OH-THC (see. 11-OH-THC (also known as 11-hydroxy-THC) is pharmacologically active, and can induce the effect of ‘feeling high’, just as THC can. 11-OH-THC has equal potency as THC, perhaps even greater.
6 The reason for this is unknown, although one research group reported that the potentially increased potency of 11-OH-THC may be because it can enter the brain easier than THC. In their study, they observed that it crosses the blood-brain barrier easier.
7 Additionally, a research group by Aditi Das found that 11-OH-THC has a larger effect on the CB1 receptor than THC.
4
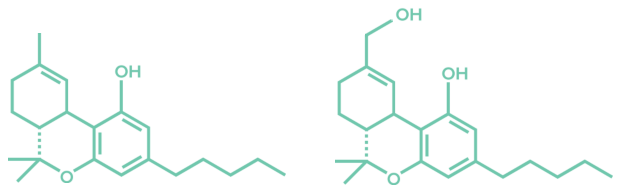 Figure 2: THC and 11-OH-THC molecules.
Figure 2: THC and 11-OH-THC molecules.
Next in the metabolic path, 11-OH-THC is converted into 11-COOH-THC (also known as 11-carboxy-THC). 11-COOH-THC does not give these prior effects. Finally, 11-COOH-THC is converted into 11-COOH-THC glucuronide, after which it is
excreted and leaves the body. Figure 3 illustrates this concept.
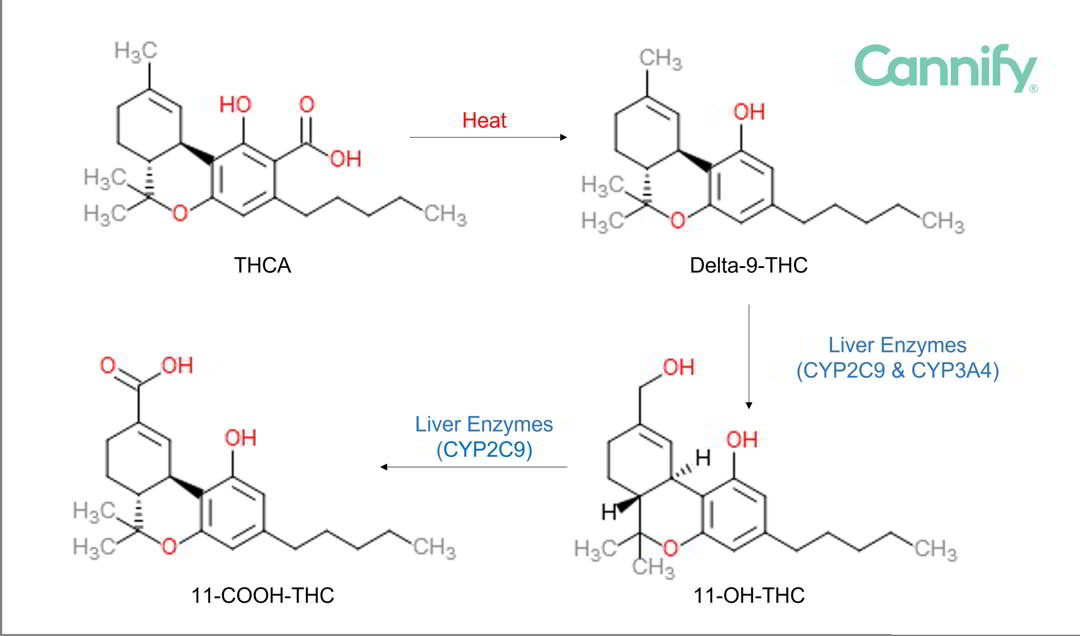 Figure 3: Metabolic pathway with structural formulas. The first step describes the conversion from the raw plant form of THC, which is THCA (THC acid) to THC by heat, called decarboxylation.
Figure 3: Metabolic pathway with structural formulas. The first step describes the conversion from the raw plant form of THC, which is THCA (THC acid) to THC by heat, called decarboxylation.
Another important factor in the metabolism of THC is that THC experiences the first-pass effect when orally administered. When THC is inhaled by either smoking or vaporizing, it avoids the first-pass effect, as described in the
First-pass effect section above. In this case, THC is absorbed directly into the bloodstream from the lungs. However, when ingested, THC undergoes the first-pass effect. The THC metabolites that are seen in the body after administration, are largely dependent on the administration route. These different ratios can have implications for the strength of the drug’s effects. For example, when administered orally (compared to being inhaled), a greater portion of the original THC is metabolized into 11-hydroxy-THC, which can produce more potent effects.
8
After being administered orally in humans,
cannabidiol (CBD) is mainly metabolized by cytochrome P450 enzymes CYP2C19 and CYP3A4. This occurs mainly in the liver, and also in the gut.
9
After CBD enters the body, it is converted into many metabolites as the body processes the drug. Some major metabolites include 7-OH-CBD and 7-COOH-CBD,
9 as well as 2”-OH-7-COOH,3”,4”,5”-trinor, CBD-glucuronide, and 4”-OH-7-COOH.
10
However, a lot of information is still unknown about the metabolism process of CBD. With the growing popularity of CBD, this is an area that needs better scientific research. Although several human studies have been done with CBD, and levels of CBD have been measured in the blood, it is hard to develop methodologies for determining the levels of the metabolites such as 7-OH-CBD in the blood. Due to these practical difficulties, we still do not know a lot about CBD metabolism in humans, but also other factors play a role, such as governmental regulations. The effects of the metabolites on our behavior or health are also unknown.
Watch a brief summary video on
cannabis inhalation vs. cannabis ingestion made in collaboration with
Enlighten on
YouTube or on
the screens page.
- Rang, Humphrey; Ritter, James; Flower, Rod; Henderson, Graeme (2015). Rang & Dale's Pharmacology (116). Elsevier.
- Dinis-Oliveira, Ricardo Jorge (2016). Metabolomics of Δ 9 -tetrahydrocannabinol: implications in toxicity. Drug Metabolism Reviews, 48(1), 80--87.
- Watanabe, Kazuhito; Yamaori, Satoshi; Funahashi, Tatsuya; Kimura, Toshiyuki; Yamamoto, Ikuo (2007). Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sciences
- Das, A. (2019). Personal communication to L. Klumpers.
- Morgan, Celia J. A.; Schafer, Gráinne; Freeman, Tom P.; Curran, H. Valerie (2010). Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. British Journal of Psychiatry, 197(04), 285--290.
- Lemberger, Louis; Martz, Robert; Rodda, Bruce; Forney, Robert; Rowe, Howard (1973). Comparative pharmacology of Delta9-tetrahydrocannabinol and its metabolite, 11-OH-Delta9-tetrahydrocannabinol. The Journal of clinical investigation (2411--7).
- Schou, J.; Prockop, L. D.; Dahlstrom, G.; Rohde, C. (1977). Penetration of delta-9-tetrahydrocannabinol and 11-OH-delta-9-tetrahydrocannabinol through the blood-brain barrier. Acta pharmacologica et toxicologica, 41(1), 33--38.
- Huestis, M. A. (2005). Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handbook of experimental pharmacology (657--90).
- GW Pharmaceuticals. Epidiolex (cannabidiol) (14). Food and Drug Administration website.
- Ujváry, István; Hanuš, Lumír (2016). Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis and Cannabinoid Research, 1(1), 90--101.
 Figure 1: Enzyme inhibition – Drug A and drug B competition illustration.
In the case of enzyme induction, both drug C and drug D would be able to bind to the enzyme. However, drug C would act as an enzyme inducer, and make the enzyme metabolize drug D faster than usual. In this example, even if the patient only took one pill of drug D, the patient could experience the effects of only half a pill of drug D due to the accelerated metabolic interaction with drug C.
Drug metabolic interactions can cause several different effects in a patient. You might also have heard about interactions between THC and CBD (e.g. memory-impairing effects of THC are mitigated by CBD, when both are taken together5), or about ‘the entourage effect’. These effects are not primarily caused by metabolic interaction, and are discussed in different sections on this education page.
First-pass effect
The first-pass effect is a phenomenon that occurs when a drug is orally administered. During this phenomenon, the drug will travel through the GI tract, and then to the liver where it is metabolized. After exiting the liver, the drug will finally enter the central bloodstream, which will distribute the metabolites and original drug (parent compound) throughout the body. Because of this process, the concentration of the drug and its metabolites in the bloodstream is different, compared to other administration methods. For example, taking a drug via IV (intravenous) injection immediately delivers the drugs into the body’s central bloodstream. With a drug that has been inhaled, it can immediately enter the bloodstream through the lungs. Therefore, the same dose of a drug taken through different administration methods will produce different effects.
Figure 1: Enzyme inhibition – Drug A and drug B competition illustration.
In the case of enzyme induction, both drug C and drug D would be able to bind to the enzyme. However, drug C would act as an enzyme inducer, and make the enzyme metabolize drug D faster than usual. In this example, even if the patient only took one pill of drug D, the patient could experience the effects of only half a pill of drug D due to the accelerated metabolic interaction with drug C.
Drug metabolic interactions can cause several different effects in a patient. You might also have heard about interactions between THC and CBD (e.g. memory-impairing effects of THC are mitigated by CBD, when both are taken together5), or about ‘the entourage effect’. These effects are not primarily caused by metabolic interaction, and are discussed in different sections on this education page.
First-pass effect
The first-pass effect is a phenomenon that occurs when a drug is orally administered. During this phenomenon, the drug will travel through the GI tract, and then to the liver where it is metabolized. After exiting the liver, the drug will finally enter the central bloodstream, which will distribute the metabolites and original drug (parent compound) throughout the body. Because of this process, the concentration of the drug and its metabolites in the bloodstream is different, compared to other administration methods. For example, taking a drug via IV (intravenous) injection immediately delivers the drugs into the body’s central bloodstream. With a drug that has been inhaled, it can immediately enter the bloodstream through the lungs. Therefore, the same dose of a drug taken through different administration methods will produce different effects.
 Figure 2: THC and 11-OH-THC molecules.
Next in the metabolic path, 11-OH-THC is converted into 11-COOH-THC (also known as 11-carboxy-THC). 11-COOH-THC does not give these prior effects. Finally, 11-COOH-THC is converted into 11-COOH-THC glucuronide, after which it is excreted and leaves the body. Figure 3 illustrates this concept.
Figure 2: THC and 11-OH-THC molecules.
Next in the metabolic path, 11-OH-THC is converted into 11-COOH-THC (also known as 11-carboxy-THC). 11-COOH-THC does not give these prior effects. Finally, 11-COOH-THC is converted into 11-COOH-THC glucuronide, after which it is excreted and leaves the body. Figure 3 illustrates this concept.
 Figure 3: Metabolic pathway with structural formulas. The first step describes the conversion from the raw plant form of THC, which is THCA (THC acid) to THC by heat, called decarboxylation.
Another important factor in the metabolism of THC is that THC experiences the first-pass effect when orally administered. When THC is inhaled by either smoking or vaporizing, it avoids the first-pass effect, as described in the First-pass effect section above. In this case, THC is absorbed directly into the bloodstream from the lungs. However, when ingested, THC undergoes the first-pass effect. The THC metabolites that are seen in the body after administration, are largely dependent on the administration route. These different ratios can have implications for the strength of the drug’s effects. For example, when administered orally (compared to being inhaled), a greater portion of the original THC is metabolized into 11-hydroxy-THC, which can produce more potent effects.8
Figure 3: Metabolic pathway with structural formulas. The first step describes the conversion from the raw plant form of THC, which is THCA (THC acid) to THC by heat, called decarboxylation.
Another important factor in the metabolism of THC is that THC experiences the first-pass effect when orally administered. When THC is inhaled by either smoking or vaporizing, it avoids the first-pass effect, as described in the First-pass effect section above. In this case, THC is absorbed directly into the bloodstream from the lungs. However, when ingested, THC undergoes the first-pass effect. The THC metabolites that are seen in the body after administration, are largely dependent on the administration route. These different ratios can have implications for the strength of the drug’s effects. For example, when administered orally (compared to being inhaled), a greater portion of the original THC is metabolized into 11-hydroxy-THC, which can produce more potent effects.8
_logo.svg)